Category:NMR: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
[[File:Spin_energy_split_new_colours.png|300px|thumb|Energy ''E'' against the magnetic field '''B'''''<sub>ext</sub>'' showing how the energy difference ''ΔE'' (red) between the spin up and down electrons changes with increasing magnetic field strength. This results in a slight difference in population between the up (purple) and down (green) states, the lower energy down state that is aligned with '''B'''''<sub>ext</sub>'' being more populated according to the Boltzmann distribution.]] | [[File:Spin_energy_split_new_colours.png|300px|thumb|Energy ''E'' against the magnetic field '''B'''''<sub>ext</sub>'' showing how the energy difference ''ΔE'' (red) between the spin up and down electrons changes with increasing magnetic field strength. This results in a slight difference in population between the up (purple) and down (green) states, the lower energy down state that is aligned with '''B'''''<sub>ext</sub>'' being more populated according to the Boltzmann distribution.]] | ||
Density-functional theory (DFT) can be used to calculate various nuclear properties of a crystal measurable through nuclear magnetic resonance (NMR) and associated methods. In the Projector Augmented Wave (PAW) approach, a frozen core approximation is used. The Kohn-Sham (KS) states near the nucleus are correctly described using all-electron (AE) partial waves. | |||
<div style="clear:both;"></div> | |||
==Chemical shielding== | ==Chemical shielding== | ||
[[File:Chemical_shielding.png|300px|thumb|The external magnetic field '''B'''''<sub>ext</sub>'' (purple) induces currents in the electrons in atoms. These currents (black arrows) in turn induce an opposing magnetic field '''B'''''<sub>in</sub>'' (red), reducing the magnetic field at the position of the nucleus, effectively shielding the nucleus from '''B'''''<sub>ext</sub>''.]] | [[File:Chemical_shielding.png|300px|thumb|The external magnetic field '''B'''''<sub>ext</sub>'' (purple) induces currents in the electrons in atoms. These currents (black arrows) in turn induce an opposing magnetic field '''B'''''<sub>in</sub>'' (red), reducing the magnetic field at the position of the nucleus, effectively shielding the nucleus from '''B'''''<sub>ext</sub>''.]] | ||
The chemical shift δ is a property that describes the shielding of an applied, external magnetic field '''B''' felt by a nucleus with non-zero spin. The chemical shift is the difference in chemical shielding σ relative to a reference σ<sub>ref</sub>. | |||
:<math> | |||
\delta_{ij} = \sigma_{ij}^{\mathrm{ref}} - \sigma_{ij} | |||
<math> | |||
\delta_{ij} | |||
</math> | </math> | ||
The chemical shielding tensor | The chemical shielding tensor can be calculated using the Gauge-Including PAW (GIPAW) method, using the {{TAG|LCHIMAG}} tag {{Cite|pickard:prb:2001}}{{Cite|yates:prb:2007}}. The chemical shielding is calculated via the induced current (cf. {{TAG|WRT_NMRCUR}}) {{Cite|pickard:prb:2001}}{{Cite|yates:prb:2007}} and has contributions from the plane-wave grid and one-center contributions (the induced field at the center of a PAW sphere due to the augmentation current inside that sphere). These are for individual atoms and exclude the contribution due to the augmentation currents in other PAW spheres. If two-center terms are important, e.g. for H, then they should be included using {{TAG|LLRAUG}} {{Cite|dewijs:jcp:2013}}{{Cite|dewijs:jcp:2021}}. | ||
{{ | |||
Learn [[insert|how to perform a chemical shielding calculation.]] | |||
<div style="clear:both;"></div> | |||
==Magnetic susceptibility== | ==Magnetic susceptibility== | ||
The magnetic susceptibility <math>\chi</math> is the degree of magnetization | The macroscopic magnetic susceptibility <math>\chi</math> is the degree of magnetization of a material in response to an applied magnetic field {{Cite|mauri:louie:1996}}. | ||
:<math> | :<math> | ||
\textbf{ | \textbf{B}_{\textrm{in}}^{(1)}(\textbf{G}=0) = \frac{8 \pi}{3} \chi \textbf{B} | ||
</math> | </math> | ||
where <math>\ | where <math>\textbf{B}</math> is the external magnetic field and <math>\textbf{B}_{\textrm{in}}^{(1)}</math> is the induced magnetic field. | ||
The orbital magnetic susceptibility is calculated via a finite-differences approach, where a key variable ''Q<sub>ij</sub>'' is approximated in two ways. The so-called ''pGv''-approximation is used by default {{Cite|yates:prb:2007}}, where ''p'' is momentum, ''v'' is velocity, and ''G'' is a Green's function. An alternative approach, the ''vGv''-approximation is available to calculate the susceptibility {{Cite|avezac:prb:2007}}, which can be switched off using {{TAG|LVGVCALC}}. With {{TAG|LVGVAPPL}} one can force VASP to use the ''vGv'' result for the <math>\mathbf{G=0}</math> contribution instead of the ''pGv'' used as default. With {{TAG|LVGVCALC}} one can suppress the calculation of the ''vGv'' magnetic susceptibility. | |||
:<math> | |||
\ | |||
</math> | |||
Like the chemical shielding, the magnetic susceptibility is calculated by linear response using {{TAG|LCHIMAG}} {{Cite|pickard:prb:2001}}{{Cite|yates:prb:2007}}, so they will both be shown in the same {{FILE|OUTCAR}} file. | Like the chemical shielding, the magnetic susceptibility is calculated by linear response using {{TAG|LCHIMAG}} {{Cite|pickard:prb:2001}}{{Cite|yates:prb:2007}}, so they will both be shown in the same {{FILE|OUTCAR}} file. | ||
Learn [[insert|how to perform a magnetic susceptibility calculation.]] | |||
<div style="clear:both;"></div> | |||
==Quadrupolar nuclei - electric field gradient== | ==Quadrupolar nuclei - electric field gradient== | ||
[[File:Quadrupolar_efg.png|300px|thumb|The quadrupolar electric field of a nitrogen nucleus coupling to electric field gradient ''V'' in MAPbI3 (methyl ammonium lead (III) iodide)]] | [[File:Quadrupolar_efg.png|300px|thumb|The quadrupolar electric field of a nitrogen nucleus coupling to electric field gradient ''V'' in MAPbI3 (methyl ammonium lead (III) iodide)]] | ||
| Line 87: | Line 49: | ||
The EFG can be calculated using {{TAG|LEFG}} {{Cite|petrilli:prb:1998}}, which also calculates ''C<sub>q</sub>'' so long as ''Q'' are defined using {{TAG|QUAD_EFG}}. | The EFG can be calculated using {{TAG|LEFG}} {{Cite|petrilli:prb:1998}}, which also calculates ''C<sub>q</sub>'' so long as ''Q'' are defined using {{TAG|QUAD_EFG}}. | ||
Learn [[insert|how to perform an electric field gradient calculation.]] | |||
<div style="clear:both;"></div> | |||
==Hyperfine coupling== | ==Hyperfine coupling== | ||
[[File:Hyperfine_coupling.png|300px|thumb|Hyperfine coupling between the nuclear spin and the electronic spin of the unpaired electron at a nitrogen-vacancy (NV) center in diamond.]] | [[File:Hyperfine_coupling.png|300px|thumb|Hyperfine coupling between the nuclear spin and the electronic spin of the unpaired electron at a nitrogen-vacancy (NV) center in diamond.]] | ||
| Line 101: | Line 65: | ||
The hyperfine tensor can be calculated using {{TAG|LHYPERFINE}} {{Cite|szasz:prb:2013}}. | The hyperfine tensor can be calculated using {{TAG|LHYPERFINE}} {{Cite|szasz:prb:2013}}. | ||
Learn [[insert|how to perform a hyperfine coupling calculation.]] | |||
<div style="clear:both;"></div> | |||
==References== | ==References== | ||
[[Category:Linear response]] | [[Category:Linear response]] | ||
Revision as of 18:23, 7 March 2025

Density-functional theory (DFT) can be used to calculate various nuclear properties of a crystal measurable through nuclear magnetic resonance (NMR) and associated methods. In the Projector Augmented Wave (PAW) approach, a frozen core approximation is used. The Kohn-Sham (KS) states near the nucleus are correctly described using all-electron (AE) partial waves.
Chemical shielding
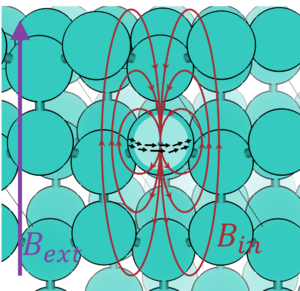
The chemical shift δ is a property that describes the shielding of an applied, external magnetic field B felt by a nucleus with non-zero spin. The chemical shift is the difference in chemical shielding σ relative to a reference σref.
The chemical shielding tensor can be calculated using the Gauge-Including PAW (GIPAW) method, using the LCHIMAG tag [1][2]. The chemical shielding is calculated via the induced current (cf. WRT_NMRCUR) [1][2] and has contributions from the plane-wave grid and one-center contributions (the induced field at the center of a PAW sphere due to the augmentation current inside that sphere). These are for individual atoms and exclude the contribution due to the augmentation currents in other PAW spheres. If two-center terms are important, e.g. for H, then they should be included using LLRAUG [3][4].
Learn how to perform a chemical shielding calculation.
Magnetic susceptibility
The macroscopic magnetic susceptibility is the degree of magnetization of a material in response to an applied magnetic field [5].
where is the external magnetic field and is the induced magnetic field.
The orbital magnetic susceptibility is calculated via a finite-differences approach, where a key variable Qij is approximated in two ways. The so-called pGv-approximation is used by default [2], where p is momentum, v is velocity, and G is a Green's function. An alternative approach, the vGv-approximation is available to calculate the susceptibility [6], which can be switched off using LVGVCALC. With LVGVAPPL one can force VASP to use the vGv result for the contribution instead of the pGv used as default. With LVGVCALC one can suppress the calculation of the vGv magnetic susceptibility.
Like the chemical shielding, the magnetic susceptibility is calculated by linear response using LCHIMAG [1][2], so they will both be shown in the same OUTCAR file.
Learn how to perform a magnetic susceptibility calculation.
Quadrupolar nuclei - electric field gradient
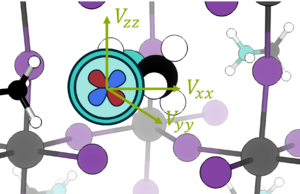
Nuclei with I > ± ½ have an electronic quadrupolar moment. This means that, at the nucleus, there is a non-zero electric field gradient (EFG) Vij, i.e. the rate of change of the electric field with respect to position is non-zero:
where V is the electrostatic potential generated by the charge distribution of electrons and nuclei.
Vij comes from the quadrupolar nuclei, which are non-spherical and therefore generate a non-uniform electric charge distribution. The electric quadrupolar moment couples with the EFG and so the chemical enviornment of the nucleus may be probed using nuclear quadrupole resonance (NQR) [7]. The EFG is not directly measurable but the nuclear quadrupolar coupling constant Cq is, defined as:
where e is the charge of an electron, Q is the isotope-specific quadrupole moment, and h is the Planck constant.
The EFG can be calculated using LEFG [8], which also calculates Cq so long as Q are defined using QUAD_EFG.
Learn how to perform an electric field gradient calculation.
Hyperfine coupling
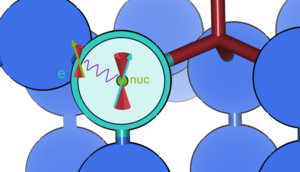
As well as the nuclei, electrons also have spin. Analogously to the nuclei, unpaired electrons can interact with Bext to provide information about their environment. The interaction between the electron's magnetic moment and the magnetic dipole moment of the nucleus (i.e. electron spin-nuclear spin interaction) split otherwise degenerate energy levels. This splitting is known as hyperfine splitting.
In most stable systems, all electrons are paired together, spin-up and spin-down, resulting in overall no spin. If a system has unpaired electrons, e.g. radicals, metal oxides, defects, then these unpaired electrons can be used to investigate these unusual systems, e.g. using electron paramagnetic resonance (EPR) [9].
The hyperfine tensor AI describes the interaction between a nuclear spin SI and the electronic spin distribution Se (in most cases associated with a paramagnetic defect state):
The hyperfine tensor can be calculated using LHYPERFINE [10].
Learn how to perform a hyperfine coupling calculation.
References
- ↑ a b c C. J. Pickard and F. Mauri, All-electron magnetic response with pseudopotentials: NMR chemical shifts, Phys. Rev. B 63, 245101 (2001).
- ↑ a b c d J. R. Yates, C. J. Pickard, and F. Mauri, Calculation of NMR chemical shifts for extended systems using ultrasoft pseudopotentials, Phys. Rev. B 76, 024401 (2007).
- ↑ F. Vasconcelos, G.A. de Wijs, R. W. A. Havenith, M. Marsman, and G. Kresse, Finite-field implementation of NMR chemical shieldings for molecules: Direct and converse gauge-including projector-augmented-wave methods, J. Chem. Phys. 139, 014109 (2013).
- ↑ G.A. de Wijs, G. Kresse, R. W. A. Havenith, and M. Marsman, Comparing GIPAW with numerically exact chemical shieldings: The role of two-center contributions to the induced current, J. Chem. Phys. 155, 234101 (2021).
- ↑ F. Mauri, S. G. Louie, Magnetic Susceptibility of Insulators from First Principles, Phys. Rev. Lett. 76, 4246 (1996).
- ↑ M. d'Avezac, N. Marzari, and F. Mauri, Spin and orbital magnetic response in metals: Susceptibility and NMR shifts, Phys. Rev. B 76, 165122 (2007).
- ↑ Nuclear quadrupole resonance, www.wikipedia.org (2025)
- ↑ H. M. Petrilli, P. E. Blöchl, P. Blaha, and K. Schwarz, Electric-field-gradient calculations using the projector augmented wave method, Phys. Rev. B 57, 14690 (1998).
- ↑ J. Weil and J. Bolton, Electron Paramagnetic Resonance: Elementary Theory and Practical Applications, (2007).
- ↑ K. Szasz, T. Hornos, M. Marsman, and A. Gali, Hyperfine coupling of point defects in semiconductors by hybrid density functional calculations: The role of core spin polarization, Phys. Rev. B, 88, 075202 (2013).
Pages in category "NMR"
The following 14 pages are in this category, out of 14 total.
